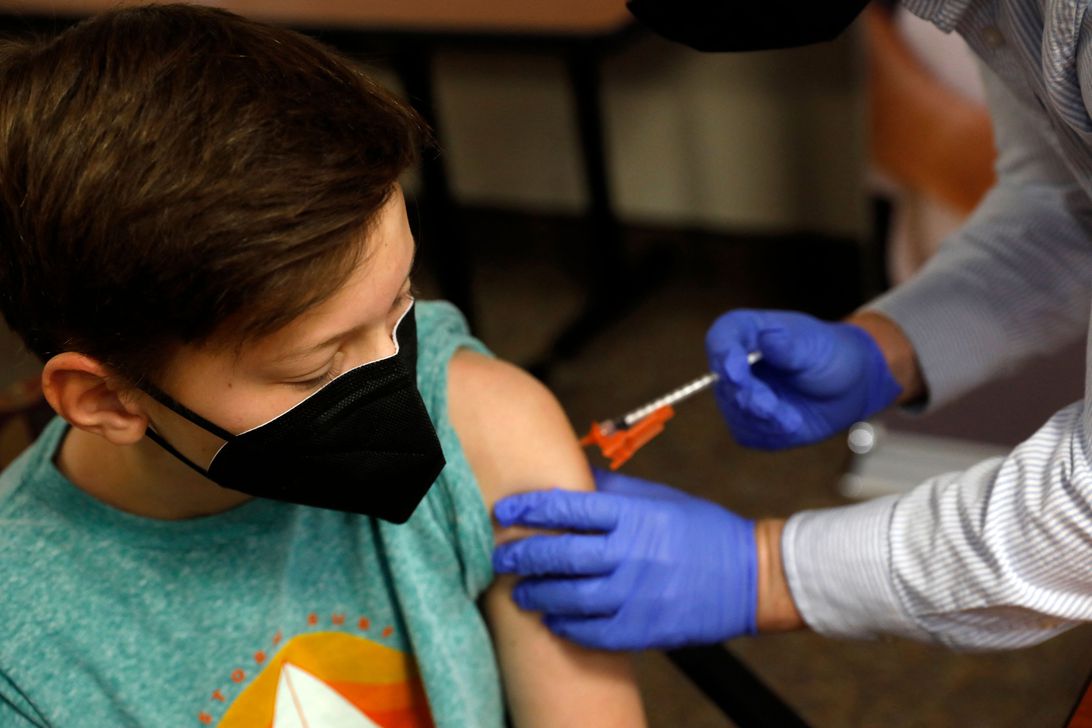
Regulators are reportedly eager to review the data from a two-dose vaccine regimen for young children, with hopes of approving an emergency authorization as early as the end of February.But data on the three-shot regimen isn’t expected to be available until late March, with regulators’ authorization expected soon after of a third dose of the pediatric vaccine.
The information contained in this article is for educational and informational purposes only and is not intended as health or medical advice. Always consult a physician or other qualified health provider regarding any questions you may have about a medical condition or health objectives.
White House medical advisor Dr. Anthony Fauci said in mid-January that a COVID-19 vaccine for children under 5 could be available by the end of February or the beginning of March. Pfizer has been testing a vaccine and booster in children ages 6 months to 5 years old and has said it expects to have more data on the results by April.
“We know that two doses isn’t enough, and we get that,” one unidentified source familiar with the situation told the Post. “The idea is, let’s go ahead and start the review of two doses. If the data holds up in the submission, you could start kids on their primary baseline months earlier than if you don’t do anything until the third-dose data comes in.”
Pfizer vaccine partner BioNTech SE is expected to ask the US Food and Drug Administration as early as Tuesday to approve emergency use of its coronavirus vaccine on children children 6 months to 5 years old, The Washington Post and The New York Times reported Monday.
CNET’s experts deliver everything you need to know to live a happy and balanced life. Delivered Thursdays.
The news comes as the more contagious omicron variant is sweeping the globe. It’s the dominant strain of COVID in the US, responsible for more than 99% of all new infections, according to the CDC. Regulators are reportedly asking for data from a two-dose vaccine regimen, potentially leading to approval of an emergency authorization within the coming weeks. The FDA approved Pfizer’s COVID-19 vaccine, Comirnaty, for all Americans ages 5 and up in October 2021.